Background: Successful treatment of pediatric acute lymphoblastic leukemia (ALL) requires 2 years of risk-directed therapy, much of which is built on a backbone of oral chemotherapy including 6-mercaptopurine (6MP) and dexamethasone (DEX). Studies conducted in the Children's Oncology Group (COG) demonstrate that poor adherence to 6MP during the maintenance phase confers increased relapse risk; however, little is known about the rates of 6MP adherence in non-COG ALL regimens. DFCI ALL Consortium protocols utilize 3-week cycles including 14-day pulses of 6MP with weekly intravenous or intramuscular methotrexate. This treatment approach requires more frequent contact between families and healthcare providers and reduces the number of oral chemotherapy agents prescribed. Whether this higher-touch regimen might reduce the frequency of non-adherence as compared to published COG data is not known. Additionally, there remains a paucity of data investigating incidence of non-adherence to DEX, 6MP, and to any medications during the first year of therapy.
Methods: Oral chemotherapy adherence was prospectively evaluated as an embedded ancillary study on the DFCI ALL Consortium Protocol 16-001. Eligible participants included English-, Spanish-, or French-speaking caregivers of patients (<22 years) receiving 6MP and DEX (both liquid and tablet formations) and did not utilize a pillbox. Adherence to 6MP and DEX were measured using Medication Event Monitoring Systems (MEMS) caps, which record the date and time of medicine bottle opening. MEMS caps were used to measure adherence for three consecutive cycles during the first year of therapy, and again for three consecutive cycles during the second year of therapy. Caregivers completed surveys reporting sociodemographic determinants of health (SDOH), medication-taking behaviors, and chemotherapy comprehension. MEMS-based medication adherence rates were calculated as a percentage based upon the ratio of the number of days with MEMS cap openings to the number of days 6MP and/or DEX were prescribed. Adherent patients were defined as those with ≥95% adherence based on prior COG trials that observed an excess risk of relapse below this threshold (Bhatia JAMA Onc 2015).
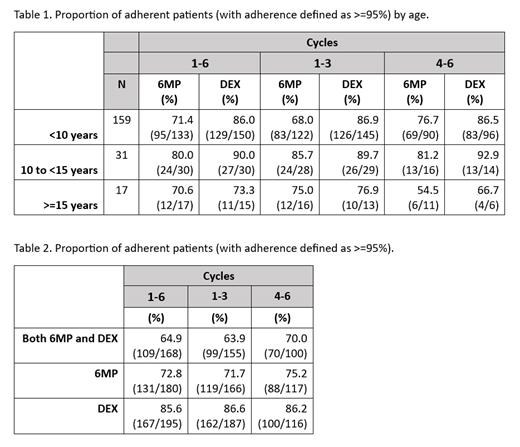
Results: Between 2018 - 2022, 252/429 (59%) eligible patients consented to study participation, and adherence data was successfully collected for 207 (82%). Sixty-six (26%) participants were enrolled from Canadian hospital centers, and 186 (74%) participants were enrolled from US hospital centers. Participant age included 159 (77%) <10 years of age, 31 (15%) 10 - 14 years, and 17 (8%) 15 - 21 years (see Table 1). Thirty-four (16%) patients identified as Hispanic, 13 (6%) non-Hispanic Black, 18 (9%) non-Hispanic Other, and 142 (69%) non-Hispanic White. 57 (31%) patients relied on public insurance at time of diagnosis, 61 (34%) patients reported a primary household language other than English, and 36 (20%) patients lived in lone-parent households. A third, 61 (34%) and 62 (34%), of caregivers reported being unsure of what would happen if their child stopped taking 6MP or DEX, respectively. Over the course of six cycles, MEMS-measured adherence rates to 6MP (N = 180) and DEX (N = 195) were 73% (range 21 - 100) and 86% (range 10 - 100), respectively. Preliminary data suggest that adherence rates to 6MP improved during year 2 of treatment (cycles 4-6 of monitoring) (Table 2). In unadjusted analyses, there was a non-significant association between patient age and likelihood of adherence to oral medication: adherence to 6MP was 71% in those <10 years, 80% in those 10 - 14 years, and 71% in those 15 years or older (Jonckheere-Terpstra test p = 0.66).
Conclusion: Among a diverse cohort of patients treated with a DFCI ALL Consortium regimen, adherence to oral chemotherapy is suboptimal with rates of 73% to 6MP and 86% to DEX. This finding recapitulates published data from COG ALL trials, suggesting more frequent contact with healthcare providers in the DFCI Consortium protocol does not mitigate non-adherence. Numerical differences between age groups suggest patients ages 10 - 14 years (N=31) have higher adherence compared to patients both younger and older. These preliminary findings suggest that there may be a behavioral component when it comes to medication adherence - with barriers among young children differing from those in both younger and older adolescents.
Disclosures
Tran:Jazz Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Silverman:Servier Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Kelly:Seagen: Other: Scientific Steering Committee; Merck: Other: Scientific Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal